
 |
U.S. Geological Survey
Water-Resources Investigations Report 03-4007
This Report has links to PDF files that can be read with Acrobat Reader. Acrobat reader is a free plug-in and can be downloaded fromAdobe. Quicktime movies are used. If you need to install the Quicktime player a Windows and a Mac version are avalable from Apple. This report is also in a pdf format 2.8Mb(without the videos).
A snow avalanche is a powerful force of nature that can play a significant role in developing mountain landscapes (Perla and Martinelli, 1975). More importantly, loss of life can occur when people are caught in the path of snow avalanches (Grossman, 1999). Increasing winter recreation, including skiing, snowboarding, snowmobiling, snowshoeing, and climbing in mountainous areas, has increased the likelihood of people encountering snow avalanches (fig. 1). Explosives are used by most ski areas and State highway departments throughout the Western United States to control the release of snow avalanches, thus minimizing the loss of human life during winter recreation and highway travel (fig. 2).
Common explosives used for snow avalanche control include trinitrotoluene (TNT), pentaerythritoltetranitrate (PETN), cyclotrimethylenetrinitramine (RDX), tetrytol, ammonium nitrate, and nitroglycerin (Perla and Martinelli, 1975). During and after snowfall or wind loading of potential avalanche slopes, ski patrollers and Utah Department of Transportation personnel deliver explosive charges onto predetermined targets to artificially release snow avalanches, thereby rendering the slope safer for winter activities. Explosives can be thrown by hand onto target zones or shot from cannons for more remote delivery of explosive charges. Hand-delivered charges typically contain about 2 pounds of TNT or its equivalent (Perla and Martinelli, 1975).
Depending on the size of the ski area, acreage of potential avalanche terrain, and weather conditions, the annual quantity of explosives used during a season of snow avalanche control can be substantial. For example, the three ski areas of Alta, Snowbird, and Brighton, plus the Utah Department of Transportation, may use as many as 11,200 hand charges per year (Wasatch Powderbird Guides, unpub. data, 1999) for snow avalanche control in Big and Little Cottonwood Canyons (fig. 3). If each charge is assumed to weigh 2 pounds, this equates to about 22,400 pounds of explosive hand charges per year. In addition, 2,240 to 3,160 Avalauncher rounds and 626 to 958 military artillery rounds (explosive mass not specified) are used each year by the three ski areas and the Utah Department of Transportation for snow avalanche control in Big and Little Cottonwood Canyons (Wasatch Powderbird Guides, unpub. data, 1999). The other ski area in Big Cottonwood Canyon, Brighton, uses about 2,000 pounds of explosives per year for snow avalanche control (Michele Weidner, Cirrus Ecological Solutions consultant, written commun., 2001).
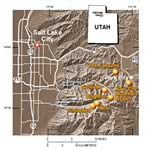 |
| Figure 3. Selected ski areas where explosives are used for snow avalanche control, Wasatch Mountains, Utah |
The use of explosives for snow avalanche control may introduce potentially toxic chemical compounds into pristine alpine and subalpine watersheds. Previous studies have shown that use of explosives and ammunition has resulted in contamination of soils, stream- and lake-bottom sediment, and aquifers with nitroaromatic compounds (Gerlach and others, 1999; Weissmahr and others, 1999). Nitroaromatic compounds, such as TNT, have potential toxic and mutagenic effects on many organisms (Stahl and Aust, 1995). To date (2002), the introduction of chemical compounds, including nitroaromatics, from explosives used for snow avalanche control has not been assessed.
The objective of this report is to determine the concentration of selected organic compounds in snow, soils, and lake-bottom sediment in areas where explosives are used for snow avalanche control in the Wasatch Mountains of Utah (figs. 4, 5, and 6).
Jenkins and others (2000) (pdf 344k) were the first to measure the concentration of explosive-residue compounds in snow. The purpose of their study was to use snow to estimate the quantity of explosive residues remaining after munitions detonation. The use of snow as a sampling medium has a number of advantages over soil sampling (Jenkins and others (2000)(pdf 344k): (1) newly fallen snow has not been contaminated from previous detonations; (2) black soot produced from the detonation allows for easy delineation of the blast zone in the snow; and (3) snow provides a convenient and relatively interference-free matrix for the chemical analysis of explosive residues. Explosive-residue compounds detected in snow samples analyzed by Jenkins and others (2000) (pdf 344k) included RDX; 2,4-Dinitrotoluene; 2,6-Dinitrotoluene; 2-Amino-4,6-Dinitrotoluene; and 4-Amino-2,6-Dinitrotoluene.
This study is the first to assess the potential effects of the use of explosives for snow avalanche control on the chemical quality of snow. Snow samples were collected from six sites (fig. 5) during February 2001. The control or background site at Summit Park is in a residential area where explosives have not been used for snow avalanche control. The remaining snow samples were collected from areas of recent and reoccurring explosive use for snow avalanche control.
Except for the background site, snow-sample sites were selected by identifying black "sooty" zones in the snow, characteristic of blast craters caused by the use of explosives (fig. 7). Prior to sampling, about 4 inches of snow was removed to expose an undisturbed sampling surface. Snow samples were then composited with a metal scoop and placed in 1-liter, cleaned and baked (450 degrees Celsius), wide-mouthed glass jars (fig. 7). The samples were kept frozen and shipped to the U.S. Geological Survey (USGS) National Water-Quality Laboratory (NWQL) in Lakewood, Colorado, for processing and analysis.
 |
| Figure 7. Black areas in snow characteristic of blast zones created by explosives used for snow avalanche control, The Canyons ski area, Utah, February 15, 2001. |
Prior to chemical extraction, the snow samples were melted at room temperature. After complete melting, two 100-milliliter (ml) aliquots were extracted, one with toluene and one with isoamyl acetate, and analyzed for explosive residues by gas chromatography/electron capture detection (GC/ECD) with established analytical techniques (Schumacher and others, 1995). The specific extraction and analytical methodology used for the snow samples is described in appendix A (PDF 123k). This extraction and analytical procedure is suitable for determination of the explosive-residue compounds listed in table 1. Most of these compounds can be detected at concentrations well below 1 microgram per liter (µg/L) in water samples.
| Compound | Lower method reporting limit for water samples, in µg/L | Lower method reporting limit for soil and sediment samples, in µg/kg |
|---|---|---|
| Nitrobenzene | < 0.05 | nd |
| 2-Nitrotoluene | < .2 | nd |
| 3-Nitrotoluene | < .2 | nd |
| 4-Nitrotoluene | < .2 | nd |
| 1,3-Dinitrobenzene | < .05 | < 2.0 |
| 2,6-Dinitrotoluene | < .01 | < 2.0 |
| 2,4-Dinitrotoluene | < .01 | < 2.0 |
| 2,3-Dinitrotoluene | < .01 | < 2.0 |
| 3,4-Dinitrotoluene (surrogate) | < .01 | nd |
| 1,3,5-Trinitrobenzene | < .1 | < 5.0 |
| 2,4,6-Trinitrotoluene | < .01 | < 2.0 |
| RDX | < .01 | nd |
| 4-Amino 2,6-Dinitrotoluene | < .05 | < 5.0 |
| 3,5-Dinitroanaline | < .02 | < 5.0 |
| 2-Amino 4,6- Dinitrotoluene | < .05 | < 5.0 |
| Tertyl | < .1 | < 5.0 |
Analysis of the snow samples resulted in detection of seven explosive-residue compounds (fig. 8). Explosive-residue compounds detected in the snow samples included 1,3-Dinitrobenzene; 2,6-Dinitrotoluene; 2,4-Dinitrotoluene; 1,3,5-Trinitrobenzene; 2,4,6-Trinitrotoluene; 4-Amino-2,6-Dinitrotoluene; and 2-Amino-4,6-Dinitrotoluene. Every snow sample collected from blast craters had at least three explosive residue compounds at detectable concentrations. Out of the seven compounds detected, 2,4-Dinitrotoluene was consistently the most abundant, with the highest concentration measured at site 3 (Park City Mountain Resort) (fig. 8). Explosive-residue compounds were not detected in the snow sample collected from the background site where explosives were not used (fig. 8).
The maximum concentration of the seven detected explosive-residue compounds was less than 1.5 µg/L (table 2). Ranges in concentrations of the detected explosive-residue compounds at the five non-background-sites are shown in table 2: 1,3-Dinitrobenzene (<0.05 to 0.08 µg/L); 2,6-Dinitrotoluene (0.05 to 0.33 µg/L); 2,4-Dinitrotoluene (0.10 to 1.30 µg/L); 1,3,5-Trinitrobenzene (detected in one sample, but not quantifiable); 2,4,6-Trinitrotoluene (<0.01 to 0.19 µg/L); 4-Amino-2,6-Dinitrotoluene (0.05 to 0.39 µg/L); 2-Amino-4,6-Dinitrotoluene (< 0.05 to 0.22 µg/L).
| Site ID | Date | Nitroben- zene | 2 Nitro- toluene | 3- Nitoro- toluene | 4- Nitoro- toluene | 1,3- Dinitro- benzene | 2,6- Dinitro- toluene | 2,4- Dinitro- toluene | 2,3- Dinitro- toluene | 1,3,5- Trinitro- benzene | 2,4,6- Trinitro- toluene | RDX | 4-Amino- 2,6- Dinitro-toluene | 3,5- Dinitro- analine | 2-Amino- 4,6- Dinitro- toluene | Tertyl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Summit Park | 2/15/01 | <0.05 | <0.2 | <0.2 | <0.2 | <0.05 | <0.01 | <0.01 | <0.01 | <0.1 | <0.01 | <1 | <0.05 | <0.2 | <0.05 | <0.1 |
| The Canyons ski area, pit composite | 2/15/01 | <.05 | <.2 | <.2 | <.2 | <.05 | .08 | .31 | <.01 | <.1 | <.01 | <1 | .09 | <.2 | <.05 | <.1 |
| The Canyons ski area, surface snow | 2/15/01 | <.05 | <.2 | <.2 | <.2 | <.05 | .19 | .56 | <.01 | E.08 | E.06 | <1 | .11 | <.2 | .11 | <.1 |
| Park City Mountain Resort, site 1 | 2/16/01 | <.05 | <.2 | <.2 | <.2 | .08 | .31 | 1.20 | <.01 | <.1 | E.19 | <1 | .39 | <.2 | .22 | <.1 |
| Park City Mountain Resort, site 2 | 2/16/01 | <.05 | <.2 | <.2 | <.2 | <.05 | .05 | .10 | <.01 | <.1 | <.01 | <1 | .05 | <.2 | <.0 | <.1 |
| Park City Mountain Resort, site 3 | 2/16/01 | <.05 | <.2 | <.2 | <.2 | .08 | .33 | 1.30 | <.01 | <.1 | <.01 | <1 | .24 | <.2 | .11 | <.1 |
The U.S. Environmental Protection Agency (USEPA) has set health advisories for four of the explosive-residue compounds detected in the snow samples from Wasatch Mountain ski areas, including 2,6 Dintrotoluene; 2,4-Dinitrotoluene; 1,3-Dinitrobenzene; and 2,4,6-Trinitrotoluene (U.S. Environmental Protection Agency, 2000). A USEPA health advisory (HA) is not a legally enforceable Federal standard, but serves as technical guidance to assist Federal, State, and local officials. Two different HA limits were used for comparison with the explosive-residue data from the snow samples: (1) 10-4 cancer risk, which is the concentration of a chemical in drinking water corresponding to an estimated cancer risk of 1 in 10,000; and (2) lifetime HA, which is the concentration of a chemical in drinking water that is not expected to cause any adverse noncarcinogenic effects for a lifetime of exposure (U.S. Environmental Protection Agency, 2000) (PDF 857k). Although some of the snow will eventually be used for drinking water, dilution of the explosive-residue compounds by mixing with uncontaminated water will likely decrease the concentrations well below analytical detection limits.
Concentrations of the explosive-residue compounds in snow samples did not exceed the 10-4 cancer risk threshold or the lifetime HA (fig. 9). Both 2,4- and 2,6-Dinitrotoluene are classified by the USEPA as probable human carcinogens and their 10-4 cancer risk threshold in drinking water is 5 µg/L (U.S. Environmental Protection Agency, 2000) (PDF 857k). The highest concentration of 2,4-Dinitrotoluene was 3.7 µg/L less than the 10-4 cancer risk threshold and the highest concentration of 2,6-Dinitrotoluene was 4.7 µg/L less than the 10-4 cancer risk threshold (fig. 9). Lifetime HA limits have not been established for 2,4- and 2,6-Dinitrotoluene concentrations in drinking water.
Only two of the six snow samples had measurable concentrations of 1,3-Dinitrobenzene or 2,4,6-Trinitrotoluene (fig. 9). The measurable concentrations of 1,3-Dinitrobenzene were about one order of magnitude less than the lifetime HA of 1 µg/L (fig. 9). The measurable concentrations of 2,4,6-Trinitrotoluene were about one order of magnitude less than the lifetime HA of 2 µg/L and about three orders of magnitude less than the 10-4 cancer risk threshold (fig. 9). A 10-4 cancer risk threshold has not been established for 1,3-Dinitrobenzene.
Overall, substantially lower concentrations of the explosive-residue compounds may be expected in snowmelt, resulting from the mixing with meltwater derived from snow that is not associated with avalanche control operations. One exception to this could be the process of preferential elution of selected ions during initial melting of a snowpack. During the initial period of snowmelt, meltwater can contain initial inorganic solute concentrations that are 12 times higher than the average concentration in the snow (Williams and Melack, 1991). Preferential solute removal from a snowpack has not been documented for explosive-residue compounds; however, if active, preferential elution could substantially increase the concentration of explosive-residue compounds in early season meltwater.
Annual snow-avalanche control operations at these locations creates the potential for an accumulation of explosive-residue compounds on surface soils. In addition, bottom sediment in streams and lakes that receive meltwater from areas where explosives are used also could act as a sink for explosive-residue compounds. Haderlein and others (1996) have shown that selected explosive-residue compounds, such as TNT, can strongly adsorb to clay minerals. This process could potentially accumulate selected explosive-residue compounds in soils and bottom sediment in areas where snow-avalanche control operations occur many times on an annual basis.
Soil and bottom-sediment samples were collected from five sites (fig. 6) during June 2001. Samples were collected after the snow had melted from the sites and about 2.5 months after any snow avalanche control had occurred with explosives. Bottom-sediment samples were collected from Shadow and Secret Lakes. Both Shadow and Secret Lakes receive runoff from adjacent slopes where explosives are used for snow avalanche control. Surface-soil samples were collected from McConkeys Bowl in areas where explosives are used for snow avalanche control.
Soil and bottom-material samples were composited with a metal scoop and placed in 1-liter, cleaned and baked (450 degrees Celsius), wide-mouthed glass jars. The samples were kept chilled and shipped to the USGS NWQL in Lakewood, Colorado, for processing and analysis. Details on the processing and analysis of the soil and bottom-material samples are reported in appendix B (PDF 66k).
The chemical analysis of the soil and bottom material resulted in the detection of three explosive-residue compounds (table 3). No explosive-residue compounds were detected in the bottom-sediment samples; however, both soil samples contained detectable amounts of explosive-residue compounds that included 2,4-Dinitrotoluene; Trinitrotoluene; and 4-Amino-2,6-Dinitrotoluene (table 3).
| Site ID | Sample medium | Date | 1,3- Dinitro- benzene | 2,6- Dinitro- toluene | 2,4- Dinitoro- toluene | 2,3- Dinitoro- toluene | Trinitro- benzene | Trinitro- toluene | 4-Amino- 2,6- Dinitro- toluene | 3,5- Dinitro- analine | 2-Amino- 4,6- Dintro- toluene | Tetryl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shadow Lake Site 1 | Bottom | 6/28/01 | <2.0 | <2.0 | <2.0 | <2.0 | <5.0 | <2.0 | <5.0 | <5.0 | <5.0 | <5.0 |
| Shadow Lake Site 2 | Bottom Sediment | 6/28/01 | <2.0 | <2.0 | <2.0 | <2.0 | <5.0 | <2.0 | <5.0 | <5.0 | <5.0 | <5.0 |
| McConkeys Bowl Site 1 | Soil | 6/28/01 | <2.0 | <2.0 | <2.0 | <2.0 | <5.0 | <2.0 | E 2.9 | <5.0 | <5.0 | <5.0 |
| McConkeys Bowl Site 2 | Soil | 6/28/01 | <2.0 | <2.0 | 3.4 | <2.0 | <5.0 | E 5.2 | E 4.3 | <5.0 | <5.0 | <5.0 |
| Secret Lake | Bottom Sediment | 6/28/01 | <2.0 | <2.0 | <2.0 | <2.0 | <5.0 | <2.0 | <5.0 | <5.0 | <5.0 | <5.0 |
Results from this initial study have raised a number of questions about the effects of explosive-residue compounds:
—By David L. Naftz, Leslie K. Kanagy, David D. Susong, Duane S. Wydoski, and Christopher J. Kanagy
Cooperation from personnel at The Canyons ski area and Park City Mountain Resort during sample collection is appreciated.
Gerlach, R., Steiof, M., Zhang, C., Hughes, J.B., 1999, Low aqueous solubility of electron donors for the reduction of nitroaromatics in anaerobic sediments: Journal of Contaminant Hydrology, v. 36, p. 91-104.
Grossman, K., 1999, The avalanche hunters: accessed November 20, 2001, at URL http://www.discovery.com/exp/avalanche/avalanche.html.
Haderlein, S.B., Weissmahr, K.W., and Schwarzenbach, R.P., 1996, Specific adsorption of nitroaromatic explosives and pesticides to clay minerals: Environmental Science and Technology, v. 30, p. 612-622.
Jenkins, T.F., Ranney, T.A., Walsh, M.E., Miyares, P.H., Hewitt, A.D., and Collins, N.H., 2000, Evaluating the use of snow-covered ranges to estimate the explosives residues that result from detonation of Army munitions: Cold Regions Research and Engineering Laboratory Technical Report ERDC/CRREL TR-00-15, 12 p.
Perla, R.I., and Martinelli, M., 1975, Avalanche handbook: U.S. Department of Agriculture Handbook 489, 238 p.
Schumacher, J.G., Kanagy, L.K., Hockanson, E.A., DeRusseau, S.N., 1995, Hydrologic and water-quality data for the Weldon Springs ordnance works, St. Charles County, Missouri, 1992-95: U.S. Geological Survey Open-File Report 96-218, 101 p.
Stahl, J.D., and Aust, S.D., 1995, Biodegradation of 2,4,6-trinitrotoluene by the white rot fungus Phanerochaete Chrysosporium, in Biodegradation of Nitroaromatic Compounds, Spain, J.C., ed., Plenum Press, New York, p. 117-133.
U.S. Environmental Protection Agency, 2000 (PDF 857k), Drinking water standards and health advisories: EPA 822-B-00-001, 18 p.
Weissmahr, K.W., Hildenbrand, M., Schwarzenbach, R.P., and Haderlein, S.B., 1999, Laboratory and field scale evaluation of geochemical controls on groundwater transport of nitroaromatic ammunition residues: Environmental Science and Technology, v. 33, p. 2593-2600.
Williams, M.W., and Melack, J.M., 1991, Solute chemistry of snowmelt and runoff in an alpine basin, Sierra Nevada: Water Resources Research, v.27, no. 7, p. 1575-1588.
For more information about the USGS in Utah go to the Utah home page
| AccessibilityFOIAPrivacyPolicies and Notices | |
 |
|